Many farmers have used foliar fertilizers with a great deal of success. Supplementary foliar feeding is a way of supplementing the plant's supply with nutrients quickly and specifically. In addition, research has shown that foliar nutrition also promotes the absorbtion of nutrients in the soil.
There are various ways in which foliar nutrition can be done, but the following three are the most important methods and each has a different aim.
|
General foliar nutrition is used for supplementary feeding and stimulation of plant metabolism. This type generally involves foliar fertilizers with a balanced content of macro and micro nutrients in a nutrient ratio which is suitable for virtually all plants. |
|
|
Crop-specific foliar nutrition is usually used for satisfying specific nutrient requirements of various crops. For example, bitter-pit in apples is caused by a localized calcium deficiency in the fruit. Calcium applied to the soil will not cure this, as the calcium is not transported to the fruit in sufficient quantities. Calcium applied via the fruit and leaves is the only way of preventing this deficiency. |
|
|
Soil-specific foliar nutrition is aimed at solving soil-specific nutritional problems. |
The fixaton of certain nutrients in the soil is the reason for applying foliar fertilizers.
Reasons for nutrient deficiency
There are many different reasons for nutrient deficiency. For example:
- Unfavourable weather conditions may inhibit the absorbtion, transport and processing of nutrients.
- An unbalanced supply of certain nutrients reduces the absorbtion of other elements.
- Certain growth stages in the life of a cultivated plant require particularly high and often quite specific levels of nutrients. Also, an adequate supply of nutrients obtained through the roots is not guaranteed because of nutritional problems of a physiological nature.
- Even though fertilizer applied to the soil may be correctly calculated, in many cases, the plant may still suffer a nutrient deficiency, although the symptoms may often be invisible ( a so-called latent deficiency) .This can result in a considerable reduction in yield.
In the above cases, foliar nutrition is an excellent and cost effective method of ensuring the plant receives a balanced supply of nutrients, because the leaves quickly absorb the nutrients which are directly available to the plant.
Basic elements
Ninety-two natural mineral elements are known to exist, but only sixty of these have been found in plants. Of these sixty, only sixteen are considered essential for plant growth.
To be considered essential for healthy plant growth, an element must fulfill four criteria.
- It must be necessary for the plant to complete it's life cycle.
- It's action must be specific, that is, not wholly replaceable by any other element.
- It must be directly involved in the nutrition of the plant, that is, required for the action of an essential enzyme.
- It must not antagonise the toxic effect of another element.
The sixteen elements that are generally considered essential for plant growth are divided into macro-elements (those that are required in relatively large quantities) and micro-elements or trace elements (those required in considerably smaller quantities.)
Essential Elements; The lack of just one essential element can ruin the chances of a good crop, even if all the other essential nutrients are there.
Before deciding which plant nutrients should be provided for in a plant it is necessary to know the function of each element.
The macro-nutrients and micro-nutrients are almost always taken up by the plant in the form of ions, which are positively or negatively charged particles.
Macro-nutrients are those which the plants need in large quantities and consist of the following:
|
Calcium (Ca) |
|
|
Carbon (C) |
|
|
Hydrogen (H) |
|
|
Magnesium (Mg) |
|
|
Nitrogen (N) |
|
|
Oxygen (O) |
|
|
Phosphorus (P) |
|
|
Potassium (K) |
|
|
Sulphur(S) |
Micro-nutrients consist of the following:
|
Boron(B) |
|
|
Chlorine(Cl) |
|
|
Copper(Cu) |
|
|
Iron(Fe) |
|
|
Manganese(Mn) |
|
|
Molybdenum(Mo) |
|
|
Zinc(Zn) |
Requirements and signs of deficiencies
The following table outlines the plant requirements for trace elements and the problems that arise when these are lacking. Each of these seven essential micro-nutrients are found in TRELMIX TRACE ELEMENT SOLUTION in the percentages shown.
|
BORON(B) 0.111% |
|
|
PLANT REQUIREMENTS |
DEFICIENCY SYMPTOMS |
|
A lack of boron in a plant results in:
The crops most susceptible to a deficiency are: cabbages, broccoli, Brussel sprouts, cauliflower, carrots, radish and citrus. |
|
COPPER(Cu) 0.319% |
|
|
PLANT REQUIREMENTS |
DEFICIENCY SYMPTOMS |
|
A lack of copper in a plant results in:
|
|
IRON(Fe) 2.260% |
|
|
PLANT REQUIREMENTS |
DEFICIENCY SYMPTOMS |
|
A lack of iron in a plant results in:
|
|
MANGANESE(Mn) 0.290% |
|
|
PLANT REQUIREMENTS |
DEFICIENCY SYMPTOMS |
|
A lack of manganese in a plant results in:
|
|
MOLYBDENUM (Mo) 0.032% |
|
|
PLANT REQUIREMENTS |
DEFICIENCY SYMPTOMS |
|
A lack of molybdenum in a plant results in:
|
|
ZINC(Zn) 0.244% |
|
|
PLANT REQUIREMENTS |
DEFICIENCY SYMPTOMS |
|
|
|
MAGNESIUM(Mg) 0.031% |
|
|
PLANT REQUIREMENTS |
DEFICIENCY SYMPTOMS |
|
A lack of magnesium in a plant results in:
|
Key to the Classical symptoms of nutrient deficiencies
|
Deficiency Symptoms |
Deficient Nutrient |
|
|
( I ) |
The dominant symptom is chlorotic foliage |
|
|
(1) |
Entire leaf blades are chlorotic |
|
|
(1,1) |
Only the lower leaves are chlorotic followed by necrosis and leaf drop |
Nitrogen |
|
(1,2) |
Leaves on all parts of the plant are affected and sometimes have a beige case |
Sulphur |
|
(2) |
Yellowing of leaves takes form of interveinal chlorosis |
|
|
(2,1) |
Only recently mature or older leaves exhibit interveinal chlorosis |
Magnesium |
|
(2,2) |
Only younger leaves exhibit interveinal chlorosis. This is the only symptom |
Iron |
|
(A) |
In addition to interveinal chlorosis on young leaves, grey or tan necrotic spots develop in chlorotic areas |
Manganese |
|
(B) |
While younger leaves have interveinal chlorosis, the tips and lobes of leaves remain green followed by veinal chlorosis and rapid, extensive necrosis of leaf blade |
Copper |
|
(C) |
Young leaves are very small and sometimes missing leaf blades altogether and internodes are short giving a rosette appearance |
Zinc |
|
( II ) |
Leaf chlorosis is not the dominant symptom. |
|
|
(1) |
Symptoms appear at the base of the plant |
|
|
(1,1) |
At first, all leaves are dark green and then growth is stunted. Purple pigment often develops in leaves, particularly older leaves |
Phosphorus |
|
(1,2) |
Margins of older leaves become chlorotic and then burn, or small chlorotic spots progressing to necrosis appear scattered on leaf blades |
Potassium |
|
(2) |
Symptoms appear at top of plant |
|
|
(2,1) |
Terminal buds die giving rise to a witch's broom. Young leaves become very thick and leathery |
Boron |
|
(2,2) |
Margins of young leaves fail to form, sometimes yielding strap-leaves. Growing point ceases to develop, leaving a blunt end. Light green colour or uneven chlorosis of young tissue. Root growth is poor in that roots are short and thickened |
Calcium |
Trace element deficiency table
|
PLANT |
ZINC |
IRON |
MANGANESE |
MOLYBDENUM |
COPPER |
BORON |
|
Alfalfa |
Low |
Medium |
Medium |
Medium |
High |
High |
|
Apples |
High |
- |
High |
Low |
Medium |
High |
|
Asparagus |
Low |
Medium |
Low |
Low |
Low |
Low |
|
Barley |
Medium |
High |
Medium |
Low |
High |
Low |
|
Beans |
High |
High |
High |
Low |
Low |
Low |
|
Broccoli |
- |
High |
Medium |
Medium |
Medium |
Medium |
|
Cabbage |
- |
Medium |
Medium |
High |
Medium |
High |
|
Carnation |
Low |
Low |
Low |
High |
High |
Medium |
|
Carrots |
Low |
- |
Medium |
Low |
High |
Medium |
|
Cauliflower |
- |
High |
Medium |
High |
Medium |
High |
|
Celery |
- |
- |
Medium |
Low |
Medium |
High |
|
Chrysanthemum |
Low |
Medium |
Medium |
Low |
High |
Medium |
|
Clover |
Medium |
- |
Medium |
High |
Medium |
Medium |
|
Cotton |
High |
- |
- |
Low |
Medium |
Medium |
|
Cucumber |
Low |
- |
High |
Medium |
Medium |
High |
|
Grapefruit |
High |
High |
High |
Medium |
High |
Medium |
|
Grapes |
Low |
High |
High |
Low |
- |
Medium |
|
Lettuce |
Low |
- |
High |
High |
High |
Medium |
|
Maize |
High |
Medium |
Low |
Low |
Medium |
Low |
|
Oats |
Low |
Medium |
High |
Medium |
High |
Low |
|
Orange |
High |
High |
High |
Medium |
High |
Medium |
|
Peaches |
High |
- |
High |
Low |
Medium |
Medium |
|
Pears |
Medium |
- |
- |
Low |
Medium |
Medium |
|
Peas |
Low |
- |
High |
Medium |
Low |
Low |
|
Potato |
Medium |
- |
Medium |
Low |
Low |
Low |
|
Radish |
- |
- |
High |
Medium |
Medium |
Medium |
|
Raspberries |
- |
High |
High |
Low |
- |
Medium |
|
Rice |
Medium |
High |
Medium |
Low |
Low |
Low |
|
Roses |
- |
High |
High |
Low |
- |
High |
|
Sorghum |
High |
High |
High |
Low |
Medium |
Low |
|
Soybean |
Medium |
High |
High |
Medium |
Low |
Low |
|
Spinach |
- |
High |
High |
High |
High |
Medium |
|
Strawberries |
- |
High |
High |
- |
Medium |
Medium |
|
Sweetcorn |
High |
Medium |
Medium |
Low |
Medium |
Low |
|
Tomato |
Medium |
High |
Medium |
Medium |
Medium |
High |
|
Turnip |
- |
- |
Medium |
Medium |
Medium |
High |
|
Wheat |
Low |
Low |
High |
Low |
High |
Low |
The influence of the least nutrient
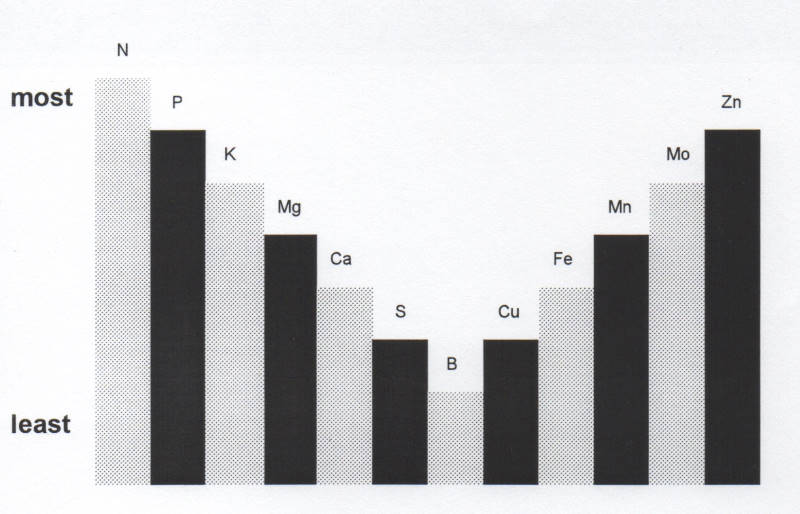
No matter how much the other plant nutrients are poured into the soil (illustrated by the heights of the columns), only as much as the level of the least ( Boron in this case) will be available to the plant.
So in order for the plant to take up all the N, P and K and other elements, the level of B, Ca, Cu, etc. must be brought up to the same level, otherwise only the quantity up to the level of the least element will be available.
All the plant nutrients placed in any growing medium can only be the equivalent of the level of the lowest element available for the production of a crop.
Put simply, pH is a measure of the acidity or alkalinity on a scale of 1 to 14. A reading of 7 is deemed to be neutral, alkaline if above and acidic if below. The following diagram gives an idea of pH values for a range of commonly found liquids.
pH values for commonly used liquids.
|
pH |
||
|
battery acid |
<1 |
|
|
vinegar |
<3 |
acidic |
|
orange juice |
>4 |
|
|
boric acid |
5 |
|
|
milk |
<7 |
|
|
pure water |
7 |
neutral |
|
blood |
>7 |
|
|
sea water |
8 |
|
|
borax |
>9 |
|
|
ammonia |
>11 |
alkaline |
|
bleach |
>12 |
|
|
lye |
<14 |
The pH value of soil, as a general rule, ranges from about 4,0 to about 8,5. An azalea, for instance, enjoys a pH of between 4,5 and 5,5 . At pH 4,5 or less, there is damage to root membranes and less favourable conditions for growth of beneficial bacteria.
Optimum pH values for some other plants:
- Camelias 5,5 to 6,5
- Gardenias 6,0
- Magnolias 6,5 to 7,0
- Hydrangeas 4,5 to 6,0 ( the lower value gives a blue colour, the higher value gives a pink colour.)
The effect of pH on the availability of nutrient elements
The pH value of a particular soil plays an important role in the availability of micro-nutrients, as the following table illustrates:
Availability of Nutrient Elements
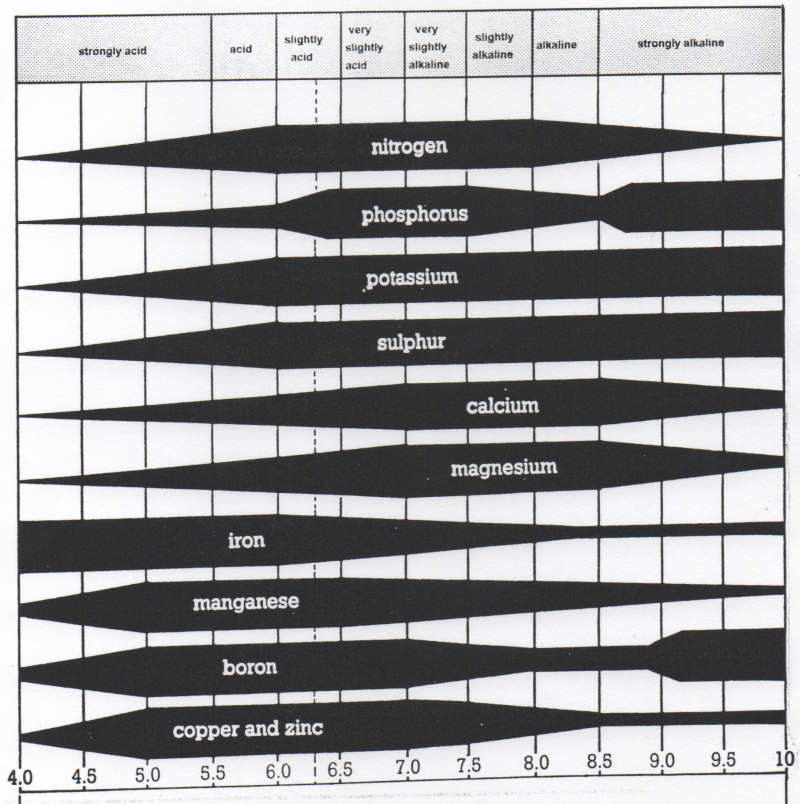
Nutrient deficiencies will become apparent if the pH is higher or lower than the recommended pH range for individual plants. For example, if the pH is consistently 7.5, one can expect interveinal chlorosis to occur, an indication of an iron deficiency.
The chart shows a pH range of 4.00 to 10.00. The width of the black section for each nutrient represents the maximum availability of that nutrient. The widest place denotes the most availability. The narrowest part denotes the least availability. The dotted line at pH 6.25 indicates the maximum number of elements at their highest availability.
Other phenomena that affect the absorption of nutrient ions
ANTAGONISM
Antagonism is the phenomenon whereby the effect of one nutrient ion reduces the effect of another.
This mostly happens at the uptake stage when the plant absorbs certain nutrients of a similar chemical property, or by the same mechanism, and one nutrient displaces another.
OK, Ca, and Mg are to be rated alike from the biochemical aspect regarding their volume and behaviour in the soil as in the plant, so these ions impede one another in the uptake and translocation of nutrients in the plant.
The same applies to Fe, Mn and Zn which also compete with each other for the nutrient uptake into the plant.
FIXATION
Fixation is the conversion of mobile nutrients into an immobile form, which is not available to the plant.
The pH of the soil can affect this and many farmers have seen how phosphorus (P) is fixed in acid soils through the formation of insoluble aluminium or ion phosphates.
An excess supply of the same nutrients can also cause other elements to be fixed.